ARECANUT
Areca catechu Linn. is commonly known as arecanut or betel nut. It is a very widely cultivated plant in eastern countries like India, Bangladesh, Ceylon, Malaya, the Philippines and Japan. The importance of this nut is due to its use for chewing purposes. Betel nut is a slender, single-trunked palm that can grow to 30 m (100 ft). The “nut” (actually the seed endosperm) is chewed as a stimulant masticatory by 5% of the world’s population, making it more popular than chewing gum but not as popular as tobacco. Use of betel nut is often culturally or socially ritualized, and there are elaborate ceremonies attending its use in various Asian and Pacific cultures. Arecanut had an important place as a pharmaceutical in Ayurveda-the ancient Indian system of medicine-also in the Chinese medicinal practices. It is used for treating leucoderma, leprosy, cough, fits, worms, anaemia, obesity and nasal ulcers. It is also used as purgative. The pharmaceutical importance of arecanut is due to the presence of an alkaloid, arecoline. Synthetic arecoline hydrobromide is also shown to possess numerous pharmacological properties. Unknown in the wild, betel nut is a cultigen that exists only where humans grow it. An origin in the Philippines has been postulated. Many other areas have been suggested as the original homeland, including South or Southeast Asia.
More than half the arecanut production in the world is from India (53%), followed by Indonesia, China, Bangladesh, Thailand and Malaysia. Arecanut palm is monoecious with male and female flowers occurring on the same spadix. The chromosome number of arecanut is 2n=32 and is a secondary allotetraploid. Seed is the only propagule of arecanut as in the case of many other palm species. This, together with the outbreeding nature of the crop, makes the populations highly heterogeneous and thereby limiting the scope of population improvement programmes in arecanut. Tissue culture seems to be the only vegetative propagation tool applicable to areca palm. Application of tissue culture technology in clonal multiplication of oil palm, date palm and coconut has been reported, but not in arecanut except for a report on adventitious shoot development from mature embryos. In vitro retrieval of arecanut embryos was reported to be successful.
The Malaya, Archipelago, the Philippines and other East Indies islands are likely to be the centre of origin since majority of the species of Areca have been reported from the said region. The genus Areca belongs to the family Arecaceae (Palme) under the tribe Areceaea and is reported to contain about 74 species. Among several species A. catechu is the only cultivated species, the nuts of which are chewed as a mild stimulant. Nuts of A. triandra are also used as a mastigatory nut. Arecanut is a graceful erect growing palm with a single unbranched stem and grows to a height of 60 to 70 feet depending on the variety and environmental conditions. The stem is smooth and is marked throughout with the scars of the fallen leaves in a regular annulated form. It has a crown of 60 to 100 pinnate leaves partly fused and partly free and with their basal region forming a sheath which completely encircles and covers the stem. The stem is cylindrical throughout and it results from a single terminal growing point situated at the top and any severe injury to buds result in the death of the palm.
In vitro Culture
The protocol for in vitro propagation via direct adventitious shoot bud differentiation from cotyledon explants. Results obtained with excised embryos of A. catechu grown on MS medium, White’s medium and Branton and Blake’s (BB) medium with permutations and combinations of auxins and cytokinins showed that activated charcoal, 2,4-D and high levels of phosphate in BB medium were critical for the differentiation of additional shoots from the cotyledon. Subsequent to germination of the excised embryo, meristemoids differentiated from single epidermal cells of the cotyledonary sheath. Differentiation of shoots along the margin of the cotyledonary sheath occurred only on separation and culture of the radicular portion of the seedling with swollen cotyledonary sheath which is attributed to the phenomenon of apical dominance in the palms. The darkening effect of activated charcoal induced rooting in shoot cultures. Synergistic action of abscisic acid and auxins in the rooting medium enhanced the frequency of rooting.
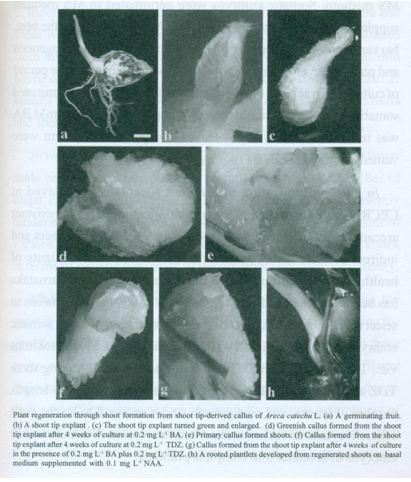 |
Plantlet formation through shoot formation from callus of Areca catechu L. has been reported. Greenish soft callus was formed from shoot tip explants of Areca catechu L. within 4 weeks, when cultured on Gelrite-gelled MS (Murashige and Skoog) basal medium supplemented with BA (0.2 mg l-1) plus TDZ (0, 0.02 and 0.2 mg l-1). The highest percentage of callus formation (100%) was found on the medium supplemented with 0.2 mg l-1 BA and 0.2 mg l-1 TDZ. During subculture on the same medium for callus induction, most of calluses proliferated and 50–60% formed shoots. About 90% of shoots formed roots on BM containing 0.1 mg l-1 NAA after 4 weeks in culture. Regeneration of plantlets from shoot tips via primary callus production and a two-step process of organogenesis, required about 20 weeks.
Plants have also been obtained through somatic embryogenesis from zygotic embryo derived callus of A. catechu. An in vitro culture procedure was established for somatic embryogenesis and plant regeneration from callus cultures. Segments of zygotic embryos were cultured on MS Basal medium supplemented with dicamba (9.05, 18.1, 27.15 and 36.3 µM). After 7 to 8 weeks in darkness wounded regions of explants formed callus with yellow, soft, glutinous structures. Proliferation and maintenance of callus was on the same dicamba containing medium. With regular subculture every 8 weeks the callus showed pale yellow, compact and nodular structures. During subculture somatic embryos were formed spontaneously from nodular callus tissues within 2 to 4 months. The embryos developed into plantlets after 10 weeks of culture on basal medium free of plant growth regulators. After subculturing every month for three months the plantlets were transferred to containers for acclimatization in the green house. The survival rate was 24 %.
Standardized with leaf explants excised from one-year-old seedlings and later modified for immature inflorescence sampled from adult palms. The basal medium used was MS. Picloram was found to be the most suitable callogenic agent for both types of explants as well as for the varieties tried. Serial transfer of explants from high to low auxin concentration was essential for sustained growth of callus and somatic embryo induction. Somatic embryogenesis was achieved in hormone-free MS medium. Somatic embryos were germinated in MS medium supplemented with Cytokinin; 20 mM BA was found to be the best. To achieve rapid growth and development of germinated somatic embryos, MS liquid medium supplemented with 5 mM BA was used. Plantlets with 2–4 leaves and good root system were veined using sand:soil (5:1) potting mixture.
In vitro multiplication of arecanut successfully achieved at CPCRI has been applied to mass multiplication of YLD resistant arecanut palm. Direct and indirect somatic embryogenesis from inflorescence explants of healthy palms identified from YLD disease hot spot of Karnataka. A study was undertaken to select the best cytokinin for conversion / maturation of direct somatic embryos into normal plantlets. Five cytokinins viz., TDZ, BA, kinetin, 2iP and zeatin were tested. Among them TDZ at a concentration of 2 mg l-1 gave maximum shoot length, number of leaves, and root growth and was found to be the most efficient cytokinin for maturation and conversion of direct somatic embryos of arecanut into complete plantlets.
Markers
Molecular markers are invaluable tools for establishing the genetic uniformity of tissue culture derived plantlets. Assessing the genetic fidelity of perennial crops is very much important, as these crops will remain in the field for a long time. Among the different molecular markers available, RAPD markers are preferred due to their cost effectiveness, technical simplicity and non requirement of sequence information of template DNA. RAPD markers were used to evaluate clonal fidelity of plantlets derived through direct somatic embryogenesis from Yellow Leaf Disease resistant arecanut palms. Pairwise genetic similarities were generated by Jaccard’s coefficient using the RAPD banding pattern between each mother palm and its progenies (eight plantlets/ palm). Mother palm and its progenies showed high similarities (99% in one case and 98% in one palm). This study showed that less variation existed in in vitro regenerated plantlets and hence in vitro regenerated plantlets derived through direct somatic embryogenesis from inflorescence culture can be employed to mass multiply elite palms with desirable qualities.
Seed is the only propagule of arecanut as in the case of many other palm species. This together with the outbreeding nature of the crop makes the populations highly heterogeneous and thereby limiting the scope of population improvement programmes in arecanut. Tissue culture seems to be the only vegetative propagation tool applicable to areca palm. The future thrust areas for palms include,
- Strengthening of germplasm collection and characterization of different traits for utilization in breeding programmes
- Cryopreservation of germplasm and development of molecular markers for marker assisted selection (MAS) in breeding programmes
- Development of regeneration protocol
- Development of theoretical ideotype for focused crop improvement
- Understanding the abiotic stress tolerance and screening germplasm for tolerance to different stresses
- Physiological and biochemical characterization for productivity potential
- Development of integrated eco friendly crop protection
- Development of databases for palms.
Source: Dr.V.Ponnuswami, PhD, PDF (Taiwan), Former Dean & Professor (Horticulture), Horticultural College & Research Institute, Tamil Nadu Agricultural University, Coimbatore
|
.png)
.png)