TISSUE CULTURE- An Introduction
Plant tissue culture is the culture and maintenance of plant cells or organs in sterile, nutritionally and environmentally supportive conditions (in vitro). Plant cell and tissue culture include the cultural techniques for regeneration of functional plants from embryonic tissues, tissue fragments, calli, isolated cells, or protoplasts. It has applications in research and commerce. In commercial settings, tissue culture is often referred to as micro-propagation, which is in fact one of the techniques in tissue culture. Micro-propagation refers to the production of whole plants from cell cultures derived from explants (the initial piece of tissue put into culture) or meristem cells.
Haberlandt was the first scientist to produce whole plants from plant tissues and so he is popularly called as the “Father of Tissue Culture’. Plant tissue culture and molecular biology form the basis for genetic engineering.
Plant tissue culture consists of pollen culture, callus tip culture, stem tip culture, meristem tip culture and protoplast fusion (Fig: 1).
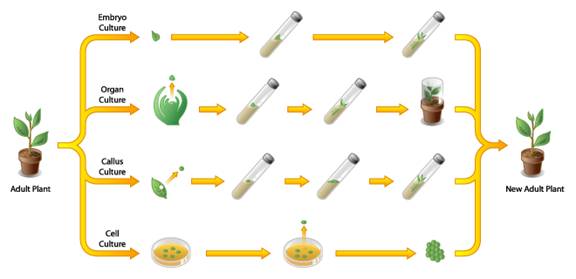
Fig: 1. Various Tissue Culture Types
Conditions required for plant cells to grow in vitro:
- Freedom from competitions particularly from microorganisms for nutrition and other factors
- Availability of nutrients and removal of waste products for better growth of the tissue
- A controlled environment to maintain the cultures
Tissue culture offers significant benefits over traditional propagation methods.
- Much faster rates of growth can be induced in vitro than by traditional means.
- Multiplication of plants which are very difficult to propagate by cuttings or other traditional methods.
- Production of large numbers of genetically identical clones in a short time
- Seeds can be germinated with no risk of damping off/ predation.
- Under certain conditions, plant material can be stored in vitro for considerable periods of time with little or no maintenance
- Tissue culture techniques are used for virus eradication, genetic manipulation, somatic hybridization and other procedures that benefit propagation, crop improvement, and basic research.
The success for plant tissue culture is based on the principle called totipotency - the ability of undifferentiated plant tissues to differentiate into functional plants when cultured in vitro. Plant tissue culture is used widely in plant science; it also has a number of commercial applications.
Applications include
- Micro-propagation is widely used in forestry and in floriculture. Micro-propagation can also be used to conserve rare or endangered plant species.
- A plant breeder may use tissue culture to screen cells rather than plants for advantageous characters such as salt tolerance.
- Large-scale growth of plant cells in liquid culture inside bioreactors as a source of secondary metabolites.
- To cross widely related species by protoplast fusion and regeneration of the novel hybrid called cybrids
- To cross-pollinate distantly related species and then rescue of the resulting embryo through culture of embryos to overcome post-zygotic abortion
- For production of doubled plants from haploid cultures to achieve homozygous lines more rapidly in breeding programmes, usually by treatment with colchicine which causes doubling of the chromosome number.
- Certain techniques such as meristem tip culture may be employed that can be used to produce clean plant material from virus infected plants.
Technique
A part or an organ of a plant is disinfected with a sterilizing agent and placed inside a test tube containing a nutrition balanced medium under controlled environments in order to culture it into a whole plant. Many explants, for example, stem, leaf, bud, meristem are used for this purpose. The figure shows a banana plantlet cultured and regenerated by means of tissue culture (Fig: 2).

Fig: 2. Tissue Cultured Banana.
By means of tissue culture it is possible to produce pathogen free plantlets by mass multiplication in a very limited amount of area from a very small sterile part of a mother plant. This method is also used to produce/ multiply plants that are to be transported across national border and so for their faster multiplication.But the establishment of a tissue culturing unit needs huge financial investments, skilled labors/technicians and required areas for its establishment are major constraints. Plant tissues grow and multiply in the labs only when there is an uncompetitive, growing condition with uninterrupted supply of nutrients.
Medium:
It contains all the elements that contribute the required nutrients that aid to the growth of the tissues; it is in liquid state or semi-solid in nature. The tissues are grown on the media. It consists of 95% of water, major and minor nutrients, plant growth hormones, vitamins, sugar rich compounds and chelating agents.
Totipotency:
It is the ability of a tissue or an organ of a plant to produce the whole plant, under the optional laboratory conditions and this is called as Totipotency. This is the baseline over which plant tissue culture relies upon.
Callus Culture:
When the cells divide into an undifferentiated mass it is called as callus. Any part of a plant can be used to produce the calli. It may be a stem, leaf, meristem or any other part. It is used to produce variations among the plantlets.
Suspension culture:
The callus produced from the explants are grown on nutrient solutions (that are semi solid) for a period of time and they are induced to produce plants with new traits.
Embryo Culture:
The method of culturing mature and immature embryos in media is called embryo culture. By this method, it is possible to produce plants from dormant seeds and seeds with metabolites that inhibit germination. This method is very important in crop improvement programs.
Somatic Embryogenesis:
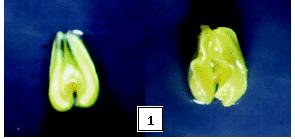
When the plants are grown on nutrient media, calli are formed. When these calli are subjected to growth in cytokinin medium, somatic embryos are formed. They are circular, elongated, heart and torpedo shaped. Among that torpedo shaped embryos produce the whole plant that is very robust (Fig: 3).

Fig: 3. Generation of Plants by Somatic Embryogenesis
- Somatic embryos
- Heart-shaped somatic embryos
- Elongated somatic embryos
- Torpedo shaped somatic embryos
- Whole plant
Apart from this above mentioned method, the suspensions of the callus are transferred to conical flasks and separate somatic embryos are produced and allowed to mature. These mature embryos when induced with cytokinin at the fourth stage produce the whole plantlets. Moreover these plants are hardened in a green house after rooting occurs. Somatic embryo culture is a very special method in plant tissue culture. Calcium alginate is used to produce artificial seeds from somatic embryos. It is very useful in studying secondary metabolites from the cell and to do research in somatic embryo culturing.
Organogenesis:
Any plant part when placed and cultured on a media, tries hard to get back its own life. In this phenomenon, the callus are produced. These callus are subjected to cytokine treatment which produce the shoots. This method of producing plant organs is called as organogenesis. These shoots are transferred to root including auxin contained media and we thus get the whole plantlets.
Embryo Rescue Culture:
Some commercially important crops hindered in germination due to the constraints related to seed anatomy and physiology. Embryo abortion is a major factor to this. These embryos of such seeds are isolated and cultured on suitable nutrient media so as to regenerate the plants easily. Embryos obtained after incompatible sexual mating also be rescued by this methodology.
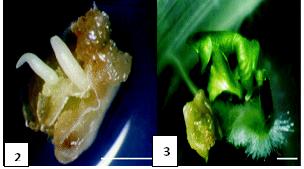
Shoot Tip Culture:
The meristem tip of a plant is more efficient in creating a whole plant than its tissues from the stem. This idea is made use of in the shoot tip culture.
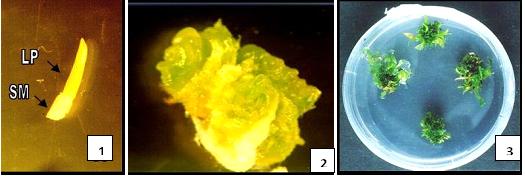

Fig: 4. Methods of Producing Plants from Shoot Tip Culture.
-
Shoot tip in the medium
-
Shoot callus
-
Growth of stem
-
Root initiation
-
Hardening
-
Whole plant
This method is used to produce plants free from pathogens, for meristems do not support the growth of viral particles. Hence the plants produced by this method can be stored pathogen free for a longer period.
Meristem Culture:
Disinfected small bits of meristem tips of 0.1 to 0.5mm length are used to produce callus on suitable media to produce whole plants. This is called as meristem culture and is used to produce pathogen free plantlets.
Anther Culture:
When the pollen/anthers of the correct stage are collected and grown on suitable media, it is called as anther culture. It is used to produce haploid plants.
-
Pollen on media
-
Callus formation
-
Shoot initiation
-
Root initiation
-
Whole plant.
The choice of mother plant for collecting the pollen, proper maintenance in lab, the pH balance of the nutrient medium, incubation period for producing the plant are all considered to be major factors.
Two Stages in Anther /Pollen Culture:
The culturing of pollen consists of two stages namely direct and indirect culturing methods. In the direct method, the pollen by themselves produce the plant directly from the medium. In the indirect method, the pollen produces a callus from which haploid plants are produced. Diploid plants are produced from the pollen sacs in a short period of time because the tips of these plants are always pathogen free.
Rejuvenation of Plants:
It is very feasible to produce the whole plant out of tissues collected from old plants in very short period of time. This is called plant rejuvenation. This method was demonstrated successfully in tapioca.
Hybrid Sorting:
It is also possible to produce hybrids from incompatible species (where in the hybrids are hard to be formed) by means of protoplasmic fusion. Hybrid may be formed by culturing the fused protoplasts in suitable nutrient media.
Micro Propagation:
The plant tissues from shoot nodes are used in plant tissue culture to produce thousands clones of a mother plant in a very short period of time (Fig: 6).
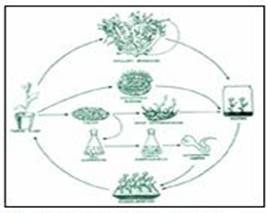
Fig: 6. Production of Plants through Mass Multiplication.
Synthetic Seeds:
Embryogenesis is the basis for various technologies on biotechnology. Somatic embryos are used to produce artificial seeds. Sodium alginate and calcium chloride are used to produce the seed coats of the somatic embryos. These are capable of producing plantlets from the soil bed just like the natural seeds. By this method, it is possible to multiply clonal plants and to create variations in the genetic architecture of the tissues. This method is used to produce vegetables, especially seedless watermelon are multiplied by this method.

Fig: 7. Production of Plants from Artificial Seeds
Protoplast Culture:
The removal of cell membrane from a cell produces a protoplast. Protoplasts are used to produce variations in the morphology of leaves and flowers, ability/potential of the growth of the embryo, and enhancement of disease resistance in plants.
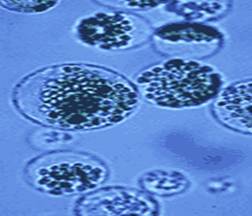
Fig: 8. Growth of Protoplast on Nutrient Media
It is feasible to produce a whole plant from a protoplast. Two protoplasts are isolated from two parents from any plant organ and are fused with chemicals that induce fusion between them. This induces variations in the genes and plants are produced with the variations.
Cryopreservation:
Embryo, shoot tip, stem tip/meristem tip and callus are used to be preserved in liquid nitrogen so as to preserve them for a longer period of time.
Content Source: CPMB, TNAU. Source for Fig: 1. http://www.jains.com/Tissue/images/tissue_main.jpg
Source for Fig: 2. http://www.scq.ubc.ca/wpcontent/uploads/2006/08/plant_cell_culture.gif
Source for Fig: 3. www.ias.ac.in/currsci/may10/articles22.htm
Source for Fig: 4. http://www.biolcell.org/boc/097/0709/boc0970709a01.gif
Source for Fig: 5. http://www.biolcell.org/boc/097/0709/boc0970709a01.gif
Source for Fig: 6. http://dbtmicropropagation.nic.in/images/inside/diagramsm.jpg
Source for Fig: 7. http://www.biology.ed.ac.uk/research/institutes/plant/pages/staff_pages/S_Millam_files/pic1.gif
Source for Fig: 8. http://whyfiles.org/128potato_blight/images/cells1.gif |