|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| | Home | Seed Village Concept | Related Links| Contact us | | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TNAU Agritech Portal :: Seed Technology | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
About Seed Seed is defined as fertilized, matured ovule consisting of an embryonic plant together with a store of food, all surrounded by a protective coat. A seed (in some plants, referred to as a kernel) is a small embryonic plant enclosed in a covering called the seed coat, usually with some stored food. It is the product of the ripened ovule of gymnosperm and angiosperm plants which occurs after fertilization and some growth within the mother plant. The formation of the seed completes the process of reproduction in seed plants (started with the development of flowers and pollination), with the embryo developed from thezygote and the seed coat from the integuments of the ovule.
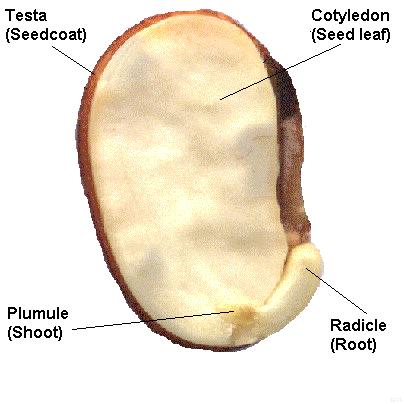
A typical seed includes three basic parts: (1) an embryo, (2) a supply of nutrients for the embryo, and (3) a seed coat.
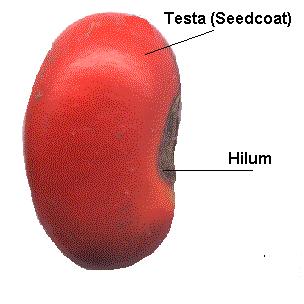
Within the seed, there usually is a store of nutrients for the seedling that will grow from the embryo. The form of the stored nutrition varies depending on the kind of plant. In angiosperms, the stored food begins as a tissue called the endosperm, which is derived from the parent plant via double fertilization. The usually triploid endosperm is rich in oil or starch andprotein. In gymnosperms, such as conifers, the food storage tissue is part of the female gametophyte, a haploid tissue. In some species, the embryo is embedded in the endosperm or female gametophyte, which the seedling will use upongermination. In others, the endosperm is absorbed by the embryo as the latter grows within the developing seed, and the cotyledons of the embryo become filled with this stored food. At maturity, seeds of these species have no endosperm and are termed exalbuminous seeds. Some exalbuminous seeds are bean, pea, oak, walnut, squash, sunflower, and radish. Seeds with an endosperm at maturity are termed albuminous seeds. Most monocots (e.g. grasses and palms) and many dicots (e.g. brazil nut and castor bean) have albuminous seeds. All gymnosperm seeds are albuminous. The seed coat (or testa) develops from the tissue, the integument, originally surrounding the ovule. The seed coat in the mature seed can be a paper-thin layer (e.g. peanut) or something more substantial (e.g. thick and hard in honey locustand coconut). The seed coat helps protect the embryo from mechanical injury and from drying out. In addition to the three basic seed parts, some seeds have an appendage on the seed coat such an aril (as in yew andnutmeg) or an elaiosome (as in Corydalis) or hairs (as in cotton). There may also be a scar on the seed coat, called the hilum; it is where the seed was attached to the ovary wall by the funiculus. Seed functionsSeeds serve several functions for the plants that produce them. Key among these functions are nourishment of theembryo, dispersal to a new location, and dormancy during unfavorable conditions. Seeds fundamentally are a means of reproduction and most seeds are the product of sexual reproduction which produces a remixing of genetic material andphenotype variability that natural selection acts on. Source : http://en.wikipedia.org/wiki/Seed#Seed_structureDifferences between seed and grain
Difference between seed and grain production
Seed is the basic input in agriculture upon which other inputs are applied. A good vigorous seed utilizes all the resources and realized a reasonable out put to the grower. It is wealth to the farmer, it is the yesterday’s harvest and tomorrows hope. Good seed in good soil realize good yield. It is a link between two generations. Seed is : 1. Carrier of new technology The introduction of quality seeds of new varieties wisely combined with other inputs significantly increases the yield levels. In India cultivation of high yielding varieties increased the food production from 52 million to 180 million tons over a period of 40 years. Seed acts as a vehicle for the superior genes to reach farmers. It is also a carrier of bio technological innovations.2. A basic tool for secured food supply Introduction of dwarf and high yielding varieties and hybrids of different crops increased the productivity and led to potential development. 3. The principal means to secure crop yields in less favourable production areas The supply of good quality seeds of improved varieties suitable to these areas is one of the few important immediate contributions to secure higher crop yields. 4. A medium for rapid rehabilitation of agriculture in cases of natural disaster Widespread floods and droughts in various parts of the country and elsewhere have focused attention on these recurrent crises and the accompanying threats of famine and starvation. The establishment of National Seed Reserve Stocks should receive high priority for meeting such natural calamities.
Goal of seed technology
In brief the role of seed technology in Agriculture sector is timely supply of quality seeds for reasonable price. Characteristics of good quality seed
These are the plants of cultivated crops found in the seed field and whose seed are so similar to crop seed that is difficult to separate them economically by mechanical means. Cause physical admixture with the crop seed only when these crop mature approximately at the same time when seed crop matures.
These are plants of weed species which are harmful in one or more of the following ways.
It refers to the diseases specified for the certification of seeds and for which certification standards must be met with. May cause contamination, when they are present in the seed field or with in the specified isolation distance in the case of loose smut of wheat. The FAO (Food and Agricultural Organization) prescribed 180 meters of isolation distance.
A true seed is defined as a fertilized mature ovule consisting of embryo, stored food material and protective coats.. The important events involved in seed development and maturation include 1. Pollination Pollination Floral biology
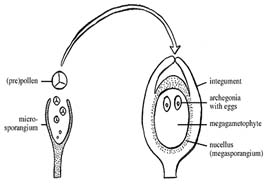
Imperfect flowers have either male (staminate flower) or female (pistillate flower) part. Such flowers are called asunisexual flowers. When both type of flowers occur in same plant – monoecious, if they occur in different plants – dioceious. Seeds usually developed from the fertilized flower for which pollination (transfer of pollen from anthers to stigma) is pre requisite. For successful fertilization viable pollen and receptive stigma are two pre requisites. The mature anthers dehisce and release pollen grains (haploid microspores). When pollen grains are transferred from an anther to the stigma of the same flower the process is called self-pollination or autogamy. If they are transferred to the stigma of another flower, cross-pollination or allogamy is said to have occurred. Self-pollination occurs in those plants where bisexual flowers achieve anther dehiscence and stigma receptivity simultaneously. The majority of angiosperms bear chasmogamous flowers i.e., flowers do not open before pollination. In some plants, flowers do not open at all such flowers is called cleistogamous, and this is the most efficient floral adaptation for promoting self-pollination. Cross-pollination is ensured in plants which bear unisexual flowers. In bisexual flowers also self-pollination may be prevented by self-sterility, dichogamy (maturation of male and female organs at different times), herkogamy (where the structure of male and female sex organs proves a barrier to self pollination) and heterostyly (where flowers are of different types depending on the length of the style and stigma and pollination occurs only between 2 dissimilar types). The important self-pollinated crops are wheat, rice, barely, mungbean and cowpea and cross pollinated are maize, rye, forage legumes and vegetables like carrot, cauliflower and onion. There is yet another category of crops called often cross pollinated crops such as cotton and pigeon pea where there may be 10-40% cross pollination. Fertilization Ovules are developed in the female gametophyte and pollen in the male gametophyte. Fertilization always takes place in female gametophyte, therefore pollen must transferred from male to female by pollen vector which may be abiotic including wind (anemophily) and water (hydrophily) or biotic including insects (entomophily) and bats (cheiropterophily). Apomixis Development of seed without fertilization i.e. the seed formation occurs without sexual fusion, the process is known as Apomixis. This occurs by several mechanism, however, all apomitic seed have genetic material only from the female plant. Apomixis may or may not require pollination and pollen tube germination to initiate seed formation, however sexual union never occurs. Parthenocarpy: Development of fruit without fertilization Seed Formation: The seed formation takes place by the following steps:
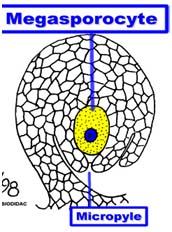
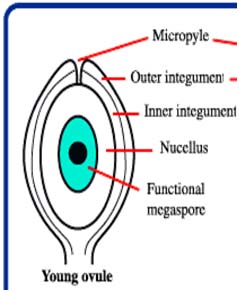
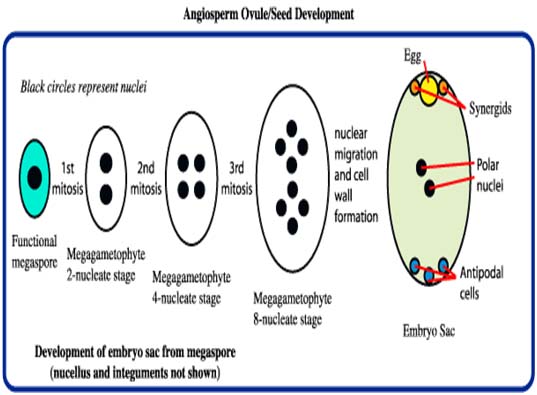
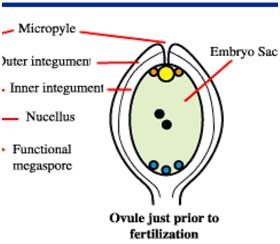
Male and female gametophyte development Female: Seeds of Angiosperm originate from meristematic tissue of the ovary wall called Ovule primordia. Within the nucellus, one cell known as Archesporial cell (2n) develops a special characteristic that distinguishes it from the adjacent cells. It develops larger than the surrounding cells, having a large nucleus and denser cytoplasm called Megaspore mother cell (MMC). MMC undergoes a meiotic division, giving rise to four megaspores , each containing a haploid chromosome set. Among the four cells one megaspore survive to give rise to anembryosac. Whereas other three aborts. Development of functional megaspore from MMC is called megasporogenesis. The nucleus within the functional magaspore undergoes three successive divisions to form eight nuclei. Eight nuclei are arranged as three antipodal cells at the chalazal end, two central polar nuclei,one egg cell with two synergids at the micropylar end. Development of eight celled embryosac from the functional magaspore is called as magagametogenesis.
Pollination
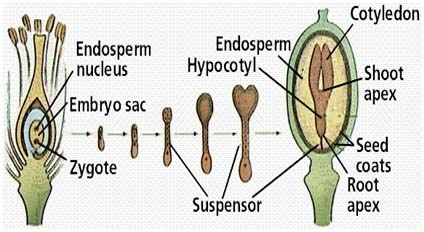
After fertilization, development of fertilized ovule into a mature seed involves several different stages. Seed formation begins within the minute embryo sac with certain expectations, which is about the same in shape, size, and arrangement. In spite of initial similarities, the seed develops according to the genetic specification for each species, which are coded in the nucleus (chromosomes) of each cell.
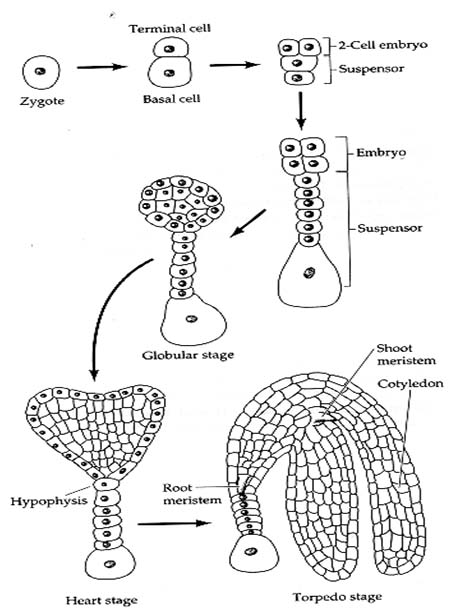
1. Embryo Development The first division of the zygote is transverse in dicots and it results in a small apical cell and a large basal cell .Cell ca divides vertically forming 2 juxtaposed cells and cb undergoes a transverse division forming 2 superimposed cells. These results in a T-shaped, 4 celled proembryo. Cell ci divides transversely giving rise to n and n'. These 2 cells divide further resulting in a row of 3 or 4 cells, forming suspensor. Cell m and its derivatives undergo vertical divisions forming a group of 4 to 6 cells. This group divides by oblique-periclinal wall forming a set of inner cells and a row of outer cells. The inner cells form the initials of the root apex and the outer cells form the root cap. The 2 cells formed as a result of the division of ca again divide vertically forming quadrant. Each cell of the quadrant divides transversely and thus an octant containing 2 tiers of cell l and p is formed. The cells of the octant undergo vertical division resulting in a globular proembryo. Periclinal divisions occur in the peripheral cells of the globular proembryo that delimit an outer layer, the dermatogen. The tier l gives rise to cotyledons and shoot apex and l forms hypocotl-radicle axis. Certain deviations from the above pattern of embryo development are found in different plants. Different types of embryogeny are distinguished depending on the plane of division of the apical and the extent of contribution of the basal cell towards embryo development (in some plants cb remains undivided and does not take part in embryo development at all). In monocotyledons, the cell cb remains undivided and develops into a haustoria of the suspension. Cell ca divides into 2 by a transverse division. The terminal cell of these 2 by repeated divisions in different planes gives rise to a single cotyledon. The embryo development in grasses is different from that of other monocotyledons. A dorsiventral symmetry is established as a result of the peculiar oblique position of cell walls early in the embryogeny. The single cotyledon is reduced to absorptive scutellum and additional structures like coleptile and coleorrhiza are formed. Development of Embryo 2. Endosperm Development There are 3 types of endosperm development (a) nuclear - where the endosperm nucleus undergoes several divisions prior to cell wall formation, e.g., wheat apple, squash, (b) cellular - in which there is no free nuclear phase and (c) helobial - where the free nuclear division is preceded, and is followed by cellularization as in some monocots. During the course of seed development, reserve food materials are accumulated in the endosperm from the adjacent tissues. In endospermic dicot seeds, endosperms are retained as a permanent storage tissue. In non-endospermic dicot seeds, endosperm reserves are depleted and occluded by the developing embryo. The reserves are then reorganized in the cotyledons, which in turn act as the source of stored reserved food for embryo after germination. A part of the endosperm is depleted in cereals during embryo maturation and this lies as a layer between the starchy endosperm and scutellum.
3. Seed-coat Development
Integuments of the ovule undergo marked reorganization and histological changes during maturation to form seed coats. In bitegmic ovules (which have 2 integuments), the seed coat may be derived from both the integuments or from the outer integument only; the inner integument may disintegrate. Seed Growth and Maturation Wheat and soybean representing monocots and dicots may illustrate the changes in the pattern of accumulation of reserve materials at different stages of seed maturation.In wheat, the dry weight of the seed increases rapidly in about 35 days after anthesis. The water content of the grain is maximum between 14 and 21 days after anthesis, and then it declines rapidly. The amounts of reducing sugar and sucrose are high between 7 and 14 days and decline rapidly thereafter due to conversion to starch.Since in wheat, starch is the major reserve material of the seed, the pattern of starch accumulation is similar to that of dry matter accumulation. The speed of germination is faster in wheat varieties that begin to lose water early during seed development. The seed is said to have physiologically matured only when it attains maximum dry weight, germinability and vigour. Normally the seed is harvested at field maturity, a stage when the moisture content is reduced to about 6-10 % in wheat. Field maturity is a crop specific character. A soybean seed attains maximum dry weight between 48 and 54 days after flowering. Oil accumulation is less during 12-18 days after fertilization; maximum oil accumulates between 24 and 42 days after flowering, after which the rate decreases. The protein content in the seed is maximum during 12-18 days after fertilization and decreases subsequently. The initial high percentage of protein may be due to the high content of non-protein nitrogen, which decreases with seed age. Oil accumulation picks up only after protein accumulation completes in the seed.
A. Innate dormancy / primary dormancy It is the state of the seed itself or dormancy induced in the seeds at the time of dispersal from the mother plant i.e. the dormancy may be induced before maturity, during maturity and after maturity but before seed is dispersed from mother plant. B. Secondary dormancy Secondary dormancy can take place only in a matured and imbibed seed by certain environmental conditions, which are unfavourable to germination. (e.g.) Spring wheat and winter barley, the secondary dormancy could be imposed by
Induction of secondary dormancy was possible one and half months after physiological maturity. Secondary dormancy in Spring wheat could not be broken by two weeks of storage. However, it was completely broken by treatment with 0.1% GA3, 0.5 to 1.0 % Ethanol, low temperature stratifications, removal of pericarp and storage at 20 0 C. Secondary Dormancy Mechanism
In many species seed dormancy is imposed by the structures surrounding the embryo (seed coat), which may include glumes, palea and lemma (grasses, the pericarp, perisperm and endosperm). The embryo in these cases are non dormant one. Primary dormancy is further classified into endogenous and exogenous . Exogenous dormancy is due to the seed coat factor either due to presence of inhibitors or hard seed nature. It is further classified into, Physical – Dormancy is due to the hard seed coat which prevents the entry of water and sometimes gaseous exchange is also prevented. e.g. Hard seeds of pulses, acacias. Prosopis, sapota etc., Chemical – Presence of some inhibitors in the seeds coat which prevents the germination Mechanical – restriction of the growth of protruding radicle due to structure. (e.g.) inadequate space in the seeds of Terminalia sp. Endogenous dormancy – Dormancy due to embryo. May be the presence of inhibitors , immature embryo or combination of both. It is further classified into Morphological – Due to immature embryo, which is not able to putforth germination even under favourable conditions . (e.g.) Apple Physiological – Due to arrest of the metabolic activity in the seeds due to presence of some inhibitors like ABA, coumarines, phenols etc., Morphophysiological – Combination of immature embryo with inhibitors. Secondary Dormancy Whose germination is inhibited , fail to recover even when the inhibitory factor is removed. Adoptive mechanism to pass the adverse environmental condition. Types of secondary dormancy Thermo – Dormancy due to temperature According to Harper (1977) dormancy may be classified into following,
Advantages of dormancy
Disadvantages
Location and cause for dormancy in certain species
Classification of seed dormancy
Dormancy breaking treatments Physical dormancy I. Scarification i. Acid ii. Mechanical iii. Physical treatment – hot water treatment 1. Scarification Any treatments may be physical or chemical that weakens or softens the seed coat is known as scarification. This method is more applicable to Malvaceae and Leguminaceae group of seeds. a) Acid scarification By using concentrated H2SO4 @ 100 ml/kg of seed for 2-3 minutes treatments dormancy can be overcome in the above group of seeds. The duration of treatment will vary and it depends on type and nature of seed coat. b) Mechanical scarification Seeds are rubbed on a sand paper or with a help of mechanical scarifier or by puncturing on seed coat with the help of needle to enhance / increase the moisture absorption by seeds. 2. Hot water treatments It is effective in case of leguminous tree crop seeds. The seeds should be soaked in boiled water for 1-5 minutes for 60-80 minutes. Some crops like Bengal gram and Groundnut, hot water treatment for more than 1 minute is found injurious to seed. 3. Stratification treatment When seed dormancy is due to embryo factor, seeds can be subjected to stratification treatments. a) Cold stratification Incubate the seed at low temperature of 0-5 0 C over a moist substratum for 2-3 days to several months. It depends on the nature of seed and kind of dormancy. (e.g.) Cherry and oil palm seeds. b) Warm stratification Some seeds require temperature of 40-50 0 C for few days e.g. paddy. In case of oil palm it requires temperature of40-50 0 C for 2 months for breaking dormancy. Care should be taken during the treatment and moisture content of seed should not be more than 15%. 4. Leaching of metabolites (Inhibitors) The seeds can be soaked in water for 3 days. But once in 12 hours fresh water should be changed to avoid fermentation or seeds can be soaked in running water for a day to leach out the inhibitors. (e.g.) Coriander (Coumarin), Sunflower (Hydrocyanic acid) 5. Temperature treatments a) Low temperature treatments Plants which grow in temperate and cooler climates, require a period of chilling for breakage of dormancy. b) High temperature treatment Normally high temperature treatments are exhibited by early flowering "winter " annuals. c) Alternate temperature treatments Most of the plant species which grow in temperate and cool temperate regions require alternate temperature for breakage of dormancy (e.g.) Bull rush (Typha). d) Fire treatment Many shrubs and trees of sub tropical and semi-arid regions have extremely hard seeds in which the seed coat is very impervious to water. Dormancy in such seeds is clearly coat imposed, and maybe broken by exposure to extreme heat such as fire. 6. Light and phytochrome
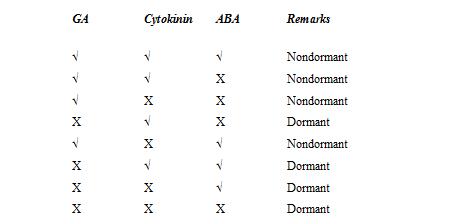
7. Promoters - inhibitors concept For regulation of germination the promoters and inhibitors present in the seed should be in a balanced manner. • GA helps in translocation of food reserve materials to active site of meristematic activity. GA also helps in cell division. • Cytokinin is a natural endogenous hormone which controls germination through DNA to RNA transcription system. • Abscisic acid is an inhibitor that can prevent germination by affecting RNA synthesis.
8. Seed Treatment with Growth Regulators/Chemicals If the endogenous dormancy is due to the presence of inhibitors, we can apply growth regulators at the low level to break dormancy.
Infra red radiation treatment Infra red rays can be passed on to the seeds and dormancy can be released. Pressure treatment Dormant seeds can be kept in autoclave and required pressure can be employed for breaking dormancy. Magnetic seed treatment Seeds can be kept in the magnetic field for about 1 to 10 days for breaking dormancy Seed Replacement Rate (SRR) Seed Replacement Rate is the percentage of area sown out of total area of crop planted in the season by using certified/quality seeds other than the farm saved seed. Seed Replacement Rates for Agricultural crops in Tamil Nadu (2008)
Source: www.seednet.gov.in The low replacement rate in groundnut indicates that farmers used the crop retained for seed purpose or obtained it from fellow farmers. However these seeds need not be of poor quality. The lateral exchange of seeds among the farmers may also help in diffusing new varieties faster. The low SRR adopted by government should be increased as proposed shown in table for proper diffusion of varieties / hybrids from seed production centres. At public sector level, the NSC, SFC and State Seed Corporations are producing quality seeds and distributing to the farming community. The quality seeds produced in government owned seed farms and farmers holdings under seed farm agreement condition are being distributed through Agricultural Extension Centres to the farming community. The seed multiplication programme is handled by the Agricultural and Horticultural Departments in their State Seed Farms. There are certain practical difficulties in the production of quality seeds in government owned farms by the Agriculture and Horticulture departments, which are now responsible for non-availability of adequate quantities of seed materials to the farmers. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| | Home | Seed Village Concept | Related Links |Contact us |
© All Rights Reserved. TNAU- 2021. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
.jpg)
.jpg)
.jpg)