|
|||||||||||||||||||||||||||||
| Fisheries :: Culture Fisheries | |||||||||||||||||||||||||||||
Seabass
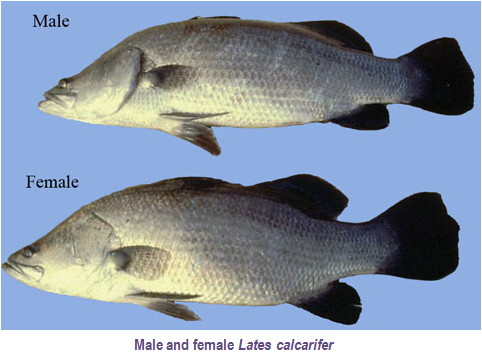
The Asian seabass (Lates calcarifer) known as "Kaalangi" or "Narimeen" in Kerala is an important candidate finfish species for farming. Seabass is a euryhaline fish, growing rapidly up to 3-5 kg within a growing period of 2-3 years in both freshwater and brackishwater environments. For maturation and spawning it migrates to the sea while the postlarvae and juveniles migrate to lagoons and backwaters for growing. It is a voracious carnivorous fish. However, the juveniles are omnivorous, feeding mainly on crustaceans and other small fishes. Seabass attains maturity at the age of 3-4 years at a length and weight range of 60 to 70 cm and 2.5 to 4.0 kg respectively. Males are generally small and in the size range of 2.0-3.0 kg and the males convert into females as they reach a size above 5.0 kg. The fecundity is between 2.1 to 17.0 million depending upon the size of the fish.
The required important facilities for a seabass hatchery complex are (a) broodstock holding tanks The saline water can either be drawn from a borewell in the intertidal area or from open sea. Water drawn should be stored in reservoirs and filtered through biological filters, rapid sand filters and ultra-violet, filter to maintain the required water quality. Broodstock development and maintenance The source of the broodstock can be either from the wild or from rearing ponds/cages. Brood fishes range in size from 2.0 to 10.0 kg, smaller size for males and larger ones for females, which can be induced to mature and spawn within 6 months. Transportation can be done using open or closed containers lined with soft materials like rubber foam mattress. Before transferring the fishes into the broodstock holding tanks (12m x 6m x 2m or 7m x 4m x 2m; rectangular in shape: with PVC inlet and outlet), they should be acclimatized in the holding tanks for. 2-3 days. Stocking Stocking of 1 kg fish biomass/m3 is recommended for a 100 tonne of water, i.e. 10 females each of average weight 6.0 kg and 16 males each of 2.5 kg. Water quality management Water exchange to an extent of 70-80% of the total volume should be done daily. A flow through arrangement for water exchange is desirable. Important parameters of water quality to be maintained are as follows: water temperature: 28-32°C; salinity: 29-32 ppt; alkalinity (CO3): 80-120 ppm; pH: 6.8-8.0; dissolved oxygen: above 5 ppm; phosphate: less than 10 ppm; unionised ammonia: less than 5 ppm; ionised ammonia: less than 1.5 ppm; Feed management Trash fishes like Tilapia/sardines can be procured, cleaned and packed as 2-3 kg blocks and frozen in deep freezers. At the time of feeding this can be thawed and given as feed to the broodstock @ 5% of the total biomass. Conditioning the broodstock fishes to feed on frozen trash fish should be done gradually, first with live and frozen fish and later with frozen fish only. Excessive feeding may be avoided and left-over feed should be removed immediately, since it will pollute the rearing medium. Health management Parasites like Caligus sp., Lernanthropus sp. are common in seabass. The most problematic parasite in seabass broodstock maintenance is the monogenic trematode Diplectanum latesi. Infected fishes do not feed, become lethargic and swim in isolation. Bath treatments with 100 ppm formalin for crustacean parasites and 1 ppm organophosphorus pesticide Dichlorovos, for 1 hr, is effective in controlling the parasites. Maturation Seabass matures spontaneously in captivity during June to October. The maturation process can be accelerated by hormonal pellet implantation. The maturity stages of the broodstock should be monitored every fortnight. For assessing the maturity stage of female, ovarian biopsy is done by catheterisation. A polythene cannula of 1.5 mm diameter is inserted into the oviduct through the genital opening and the eggs are collected. When the diameter of eggs is more than 0.450 mm in size, the fish can be induced to spawn In matured males, white creamy milt will ooze out when the abdomen is gently pressed. Induced spawning Matured seabass can be induced to spawn by hormonal manipulation. The hormones like LHRH-a (Luteinizing Hormone Releasing Hormone analogue), HCG (Human Chorionic Gonadotropin), ovaprim, ovatide and carp pituitary extract can be used for induced spawning, of which, the LHRH-a hormone is known to give consistent results. Female and male fish in the ratio of 1:2 are selected for hormone treatment. Hormone is administered normally in the early hours of the day to facilitate the spawning in the evening hours of subsequent day. Full moon or new moon days are preferred for spawning. LHRH-a hormone is administered intramuscularly in a single dose @ 60-70 mg/kg body weight for females and 30-35 mg/kg body weight for males and the fishes are released into spawning tanks of 15-20 tonne capacity. The abdominal swelling and the courtship behaviour of the fish externally indicate the ovulation response. Good water quality and aeration should be provided in the spawning tank. After 35-36 hrs of hormone injection, the fish spontaneously spawns. Seabass is a protracted spawner (i.e. ova are spawned in batches by the same fish). The same female can be induced to spawn 3 times in a season with an interval of 15 days. Fertilization is external and the fertilized eggs, which are transparent, measure 0.78-0.80 mm in diameter and float on the surface. The unfertilized opaque eggs sink to the bottom. The rate of fertilization may be 60-90%. From a single spawning, 0.75 to 1.5 million eggs can be obtained. Incubation and hatching The floating fertilized eggs can be collected from the spawning tanks by a scoop net made of bolting cloth and stocked @ 80-100 nos./ litre in cylindro-conical shaped incubation tanks of 500 litre capacity. Hatching takes place 17-18 hrs after fertilization. Newly hatched larvae measure 1.4-1.6 mm in length and the healthy ones are transferred to larval rearing tanks. The larvae and post larvae are reared at first in indoor tanks until they metamorphose into fry at about the 20th day after hatching. Larvae are stocked @ 30-40 nos./litre in fibre glass/RCC rectangular tanks of 4-5 tonne capacity. Larvae thrive on the yolk for the first 3 days. The 3-day old larvae should be fed with rotifers (Brachionus plicatilis) @ 2 nos./ml initially and gradually increased to 5 nos./ml till 8th day. From 9th day onwards, larvae should be fed with 5 nos./ml. rotifer and Artemia nauplii. Artemia nauplii density should be maintained @ 1 no./ml initially and increased to 3 nos./ml upto 15th day. From 16th day to 25th day, larvae should be exclusively fed with Artemia nauplii at a density of 4-5 nos./ml. All the feeds should be given in 4 doses at an interval of 6 hrs. After 20 days the larvae metamorphose into fry. The survival rate from hatching to fry stage is about 35-42%. Nursery rearing Nursery rearing of seabass fry in ponds and cages to stockable juvenile size is essential before release into the grow-out ponds. Nursery ponds may range in size from 500-2000 m2. A water depth of 50-80 cm is desirable. In a prepared nursery pond, fry of 1.0 to 2.5 cm size can be stocked @ 20-50 nos./m2. Water exchange to the extent of 30% is required daily. Fry must be fed with supplementary feed of chopped and ground fish (4-6 mm size) @ 100% of the body weight, twice a day, in the first week. The feeding rate is gradually reduced to 60% and 40% during second and third week respectively. Though seabass prefers live fish food it could be weaned to trash fish within 5-7 days. The nursery-rearing period is about 30-45 days. On 25th day, the fry measures 1.0 cm, which should be transferred to nursery tanks in the hatchery or nursery hapas at the farm site. Fry are stocked @ 1000 nos./m3 in 4-5 tonne capacity tanks. The cooked and minced fish meat, made into small pieces of 1.5-2.5 mm, should be given as feed ad libitum during the nursery rearing. Grading (removal of shooter fish) should be done on alternative days to reduce cannibalism. In the case of rearing seabass in the hapas (2m x 2m x 1m size), fry should be stocked @ 500 nos./m2. Feeding and grading should be done as in the case of tank rearing. The expected survival rate would be 80-86% with an average size of 1.25g in 30-35 days of rearing in the tank or hapa. Nursery cage size may range from 3 m (3x1x1 m) to 10 m (5 x 2 x 1 m) with a mesh size of 1 mm. Stocking density with fry of size range 1.0 - 2.5 cm may vary from 80-100 nos./m. Feeding schedule to be followed as in pond rearing. Cages should be checked and cleaned regularly. The fry on reaching a size of 5-10 g at the end of a rearing period of 30-45 days can be stocked in the grow-out system. Usually a survival rate ranging from 50-70% could be obtained.
Traditional culture Extensive culture of seabass as a traditional activity is followed in the Indo-pacific region. In low lying coastal ponds, juveniles of assorted sizes collected from estuarine areas are introduced and fed with the forage fishes like tilapia, shrimps and prawns available in these ponds. These ponds receive water from adjoining brackishwater or freshwater canals or from monsoon flood. Harvesting is done after 6-8 months of culture. Since seabass exhibit differential growth, the size of the harvested fishes varies from 0.5 to 5.0 kg. Production up to 2 ton/ha/7-8 months has been obtained. Cage culture The traditional culture of seabass can be improved by stocking uniform sized seed at specific density and feeding them with low cost trash fishes/ formulated feed. Water quality is maintained through periodic exchange. Fishes are allowed to grow to marketable size and harvested. Seabass culture can be done in more organized manner as a small scale/large scale activity in both brackishwater and freshwater ponds and also in cages. The size of the cage may be 50 m (5 x 5 x 2 m) with mesh size depending upon the size of the fish to be stocked (0.5 cm for 1-2 cm size fish, 1.0 cm for 5-10 cm size fish, 2.0 cm for 20-25 cm size fish and 4 cm for fish larger than 25 cm in size). In cage culture, both floating and stationary net cages are used. Floating Cages Floating cages can be set on coastal waters where tidal fluctuation is wide. The net cages are hung on GI pipe, wooden or bamboo frames. The cage is kept afloat by styrofoam drum, plastic carbuoy or bamboo. The most convenient dimension for a cage is that of a rectangle and a volume of 50 m3 (5.5m × 6m × 2m). The cage unit is stabilized with concrete weights at each bottom corner. The cage unit has to be anchored to the bottom.
Stationary Cages This type is fastened to wooden poles installed at its four corners. Stationary cages are usually set in shallow bays where the tidal fluctuation is narrow. The mesh size of nylon net would depend on the size of fish. Fingerlings should be transferred to nylon net (mesh size of 2.0 cm) for about 2 months of culture period and then they are moved to a cage net of 4.0 cm mesh size until harvest. Stocking with seed of uniform size is desirable to avoid cannibalism. The stocking density may be about 40-50 fish / m initially. However after 2-3 months of rearing, depending upon the survival rate etc., it may be reduced to 10-20 fish/m. Periodic transfer of fish from one cage to another is essential in order to grade the fish and maintain uniformity in size. Feeding schedule is similar to that in pond culture. The cages should be cleaned regularly and periodically inspected for damage. A production level up to 15-17 kg/m can be obtained in cage culture over a period of 7-8 months. Pond culture The two-week nursery reared fingerlings are suitable for pond culture. The production pond can have concrete walls and a soft bottom, ranging in area from 0.1 ha to a few ha, water depth of up to 2 m and salinity of 5-10 ppt is suitable. Seabass culture in ponds can be carried out either by polyculture method or by feeding with low cost fishes like tilapia/oil sardines or with extruded floating pellets. The pond is at first dried, tilled, leveled and manured with raw cow dung @ 1000 kg/ha. If required, lime is added @ 50-200 kg/ha to maintain soil pH above 7. Urea @ 100kg/ha and super phosphate @ 50 kg/ha can also be added to enhance the algal bloom. Sea water/fresh water is then filled to a depth of 60-70cm in the pond. When the pond water becomes light green in colour indicating sufficient development of algae in the pond, forage fishes are introduced. In pond culture, stocking with seed of uniform size (5-10 g), @ 3000-5000 nos./ha is desirable. Feeding of fish is carried out following two methods. In the first method, the fish are fed exclusively with chopped trash fish @ 10% of biomass twice daily (08.00 & 17.00 hrs) and reduced to 5% subsequently. In the other, the food is made available in the pond in the form of forage fish like Tilapia (Oreochromis mossambicus). Pelletized feed can also be given. In a well-prepared pond, manured/fertilized with raw cow dung @ 1000-1500 kg/ha and urea @ 100-150 kg/ha, Tilapia adults (male and female in the ratio 1:3) are introduced and reared for 1-2 months prior to stocking with seabass. To maintain natural food production for the forage fish, periodic manuring at fortnightly interval is done @ half the initial dose. 20% of pond water is exchanged on alternate days. Harvesting is done by draining the ponds or by using seine nets. Grow-out pond culture of seabass can yield a production of 2-3 tons/ha within a rearing period of 7-8 months. Harvesting For sea bass farmed in cages, harvesting is relatively straightforward, with the fish being concentrated into part of the cage (usually by lifting the net material) and removed using a dip net. Harvesting sea bass 'free-ranging' in ponds is more difficult, and requires seine-netting the pond or drain harvesting. After harvesting, the barramundi are placed in ice slurry to kill them humanely and preserve flesh quality. Fresh barramundi is generally transported packed in plastic bags inside styrofoam containers with ice. There is a limited market for live barramundi in Kerala. Fish are usually transported live in tanks by truck. Seabass is prone to diseases caused by parasites, bacteria, fungi and virus. But diseases and abnormalities due to environmental stresses and nutritional deficiencies have also been recognized. Viral disease Lymphocystis Disease Lymphocystis disease is commonly found in seabass raised in cages especially among juveniles 4–7 cm in total length. It has been observed at all temperatures in rather high salinity. A gross sign of the disease is massive enlargement of the cell within the dermis layer of the fish skin, which resembles the cauliflower disease. Transmission is from fish to fish. Bacterial diseases Fish reared in intensive culture conditions are exposed to extreme environmental fluctuations, and they may be more sensitive to stress than wild populations. a. Aeromonas bacteria Whenever fishes are exposed to environmental stress or injury, aeromonas causes serious outbreaks of homorrhagic disease with high mortality. Temperature, pH, high CO2 and DO depletion, decomposition products, and free ammonia in the water, all of these can be considered as possible factors for Aeromonas infection. When seabass are overcrowded and water salinity is low for long periods, the diseased fish caused by A. punctata could be observed. Gross signs and behaviours are usually shown by hemorrhage on the fin and tail. In a heavy case, erosion of tail and fin can be seen. b. Vibrio bacteria Diseases caused by Vibrio sp. typically appear as ulcerative hemorrhagic septecaemia. The typical symptoms of vibrio disease include congeston of the fins, eccymoses and petechiae on the body surface and frequently, haemorrhages and ulceration of the skin and muscle tissue. The tissues surrounding the infected anus (the vent) are usually reddened and inflamed. Internally, there is congestion and haemorrhagia of the liver, spleen and kidney, frequently accompanied by the presence of necrotic lesions. The gut and particularly the rectum may be distended and filled with a clear viscuos fluid.The body is completely covered by a thick layer of mucous. Occasionally, small-unbroken lesions are present. There may be a reddening of the caudal funs and vent. Internal organs appear normal. Young fish die more rapidly than adults.The pathogenic vibrio which have been isolated from seabass include Vibrio parahaemolyticus, V. anguillarum and V. alginolyticus. c. Columnaris disease Columnaris disease caused by Flexibacter columnaris is one of the diseases commonly found in juvenile seabass which are raised in water of low salinity during rainy and winter seasons. Gross signs are observed by saddle-shaped lesions in the mid-body position about the dorsal fin of the fish. The bilaterally symmetrical lesion appears as a fuzzy, pale yellow white plague, with dark margins, often eroding in the epidermis. Clinically, the condition may be chronic, acute or peracute. The gram negative, aerobic bacillus (about 12 um) can be isolated from the lesion of the diseased fish. Parasitic Protozoa Protozoans are probably the most important group of animal parasites affecting fish. Many reports from all over the world indicate great losses in fish culture caused by protozoans. Obligate parasites such as the ciliate ichthyophthirius and certain species of the cnidosporidians are responsible for many of these losses. Many species, which are considered as commensal protozoans, may become pathogenic under certain conditions. Environmental factors affect the susceptibility of fish to certain protozoans. Oxygen concentration and temperature are the factors affecting both hosts and parasites. Since many protozoans transfer from fish to fish through the water, fish population density is an important factor. Tremendous infestation of protozoans can occur in a relatively short time where fish populations are dense. Other factors, such as host size, age, host specificity, immunity, and the aforementioned influences of host condition also play an important part in the host reaction to invasion by protozoans. Most host reactions to invasion by protozoans are directed towards expelling or isolating the parasite. Protozoans cause harm to fish mainly by mechanical damage, secretion of toxic substance, occlusion of the blood vessels, depriving the host of nutrition and rendering the host more susceptible to secondary infections. Some of the most common clinical signs are changes in swimming habits, such as loss of equilibrium, flushing or scraping, loss of appetite, abnormal colouration, tissue erosion, excess mucous production, haemorrhage, and swollen body or distended eyes. a. Cryptocaryon sp. Cryptocaryon is a marine counterpart of the freshwater Ichthyophthirius species and similarly causes the white spot diseases in marine fish. Its morphology and life cycle is quite similar to that of the “Ich”. The surface of invaded fish reveals white pustules or numerous minute, greyish vesicles which are nests of cilliates burrowing under the epidermis. They feed on the host's cells underneath the epithelium and cause heavy irritation resulting at first in excessive production of mucous and finally completely destroying the fine respiratory platelets of the gill filaments. On the skin, this parasitic protozoan causes considerable lesions resulting in destruction of large areas of the epidermis. Secondary infection may complicate the situation and the host dies. The incidence of Cryptocaryon sp. in seabass showed a distinct peak during low water temperature period, with a marked prevalence during February. This ciliated protozoan probably causes more damage to fish populations over the entire world than any other single parasite. b. Trichodina sp. Members of the genus Trichodina with about 60 species described from marine fish and related peritrichous ciliates are the most common parasitic protozoans that are especially harmful to young fish. The species attach themselves to the gills of marine fish. More than half of juvenile seabass heavily infected with this parasite died. Trichodina also causes problems to crowded seabass in cages. Clinical signs of trichodinosis include excess mucous production, flushing, debility and hyperplasia and necrosis of the epidermis. The fin may become badly frayed in heavily infected fish and this may be accompanied by sluggishness and loss of appetite. Excessive number on the gills of infected fish interferes with respiration. c. Henneguya sp. Henneguya is a flaggelate found to attach mainly to the gills of seabass in cages. In heavy infections, it may be found in the skin. Gross signs are hyperplasia, bronchitis plus necrosis. Life cycle involves a free-swimming dinospore, which moves by means of 2 flagellae. It attaches to the host and transforms to a sac-like trophont, which has elaborate attachment mechanism. It feeds and grows and detaches from host, sinks to substrate where it encysts and produces dinospores. d. Epistylia sp. Epistylis is another protozoan found in seabass especially in freshwater environment. Epistylis belongs to the sub-class Peritrichia, and is common ectocommensals; however, it occasionally turns pathogenic. It attaches to the fish with its stalks. This protozoan may be present at a variety of temperatures and its number may be large enough to cause a grey mat on the epithelial surface. Parasitic Helminthes Worm diseases with the possible exception of those produced by monogenetic trematodes have not yet appeared to be a serious problem in seabass culture. This is probably due in large part to their complex life cycle and the difficulty in completing such cycles in the culture system. Helminthis parasites, which have been found in seabass, include monogenetic trematode, digenetic trematide, nematide and acanthocephala. a. Monogenetic trematodes Monogenetic trematodes can be observed throughout the year. Abundance of these parasites and their seasonal distribution have not been studied. It has been reported that temperature apparently plays an important role in determining outbreaks of certain parasitic Monogenera. Peak infections of monogenetic trematodes usually occur among young susceptible fish. Such behaviour is advatanegous for the spread of the organism in a fish population. b. Digenetic trematodes Lecithochirium sp. was found in the intestine of seabass especially in wild fish. Incidence of infection was 86.0 percent and average parasite burden was 5.5 Another digenetic trematode which was commonly found in the intestine of wild seabass is Pseudometadena celebesensis. Its incidence of infection and parasite burden were 100 percent and 9.3, respectively. c. Nematodes Although many species of nematodes are found either as adults or larvae in fish, few have been implicated as serious pathogens of their hosts. In seabass, nematode of the genus Cucullanus was found more common in the gut of larger fish than in that of young fish. d. Acanthocephala Acanthocephalid worms, despite their fearsome-looking proboscis with its rows of hooks, have not been observed as serious pathogens of fish. The great majority of acanthocephalus in seabass are found as adults in the gut. Parasitic Copepods The parasitic copepods are among the most devastating of fish parasites. The mature female usually attaches to the fish and feeds on the host. After copulation the female matures and produces egg sacs while the male dies. a. Caligus sp. Caligus sp. has caused big problems in cultured seabass. They attach to the gills, buccal and opercular cavities, occasionally on the skin and fins of the seabass. Heavy infections can cause mass mortalities especially in young fish. b. Lernanthropus sp. Lernanthropus are found attached to the gill of seabass especially in cage cultured fish. Large numbers of this parasite can cause anaemia to the fish host. Treatment Treatment is usually in the form of chemotherapy, possible combined with some of the preventive measures listed above. Chemical control should be a “last resort” in disease control. Chemical Prophylaxis To treat the pond accurately, the volume of water in the pond must be known. To determine the volume of a pond, multiply the number of surface unit area of water by the average depth of the pond. The following chemicals are often used in the treatment of various fish diseases:
For small ponds, dilute the chemical in a bucket of water and distribute evenly over the pond using a dipper. In large ponds, the chemical should be mixed in a large drum and distributed evenly over the pond. General Treatments When treating fish, it is advisable to know the quality of the water because such things as pH and temperature greatly affect treatment results. Treat a few fish first and see how they react before treating the entire group. Use only the drugs and chemicals that have been cleared by an authorized agency for use on food fish. Here are some general treatments for specific groups of pathogens:
Remember the following precautions during treatment:
(Source:www.vuatkerala.org) |
|||||||||||||||||||||||||||||
© TNAU 2008-2024 All Rights Reserved. |
|||||||||||||||||||||||||||||